45 fda structured product labels
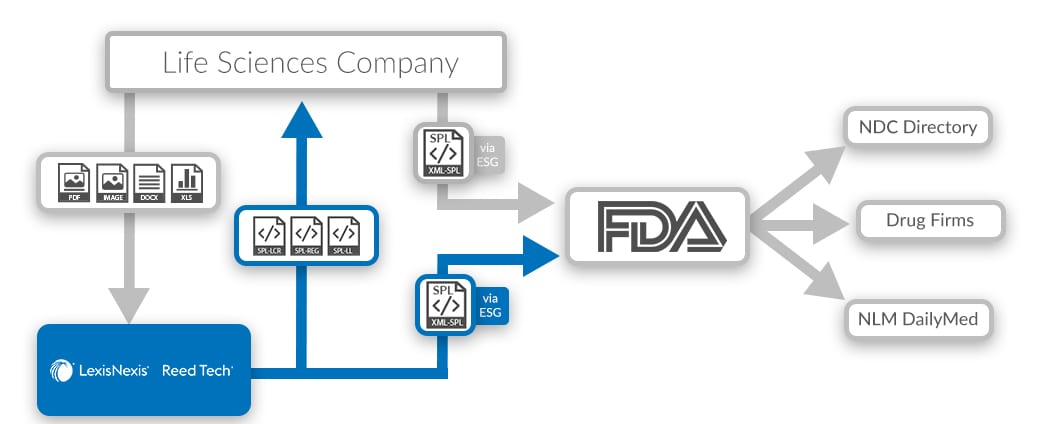
Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. RxNorm Overview - United States National Library of Medicine Jan 03, 2022 · Drugs will first need to be registered in one of the RxNorm data sources, such as FDA Structured Product Labels, to be included in RxNorm. Pharmaceutical companies that want their drugs in RxNorm should submit Structured Product Labeling (SPL) information for each product to the FDA if it has not been done already. Send questions regarding SPL ...
MTHSPL (FDA Structured Product Labels) - Synopsis SPL is a document markup standard approved by Health Level Seven (HL7) and adopted by the FDA as a mechanism for exchanging medication information. Metathesaurus Scope MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 158,821 drug products and 21,070 substances.
Fda structured product labels
Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing › documents › 2018/12/21Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. FDA SPL - Structured Product & Drug Labeling Composition Process Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product and facility information. Accepted by the Food and Drug Administration, structured product labeling enhances the cohesiveness and honesty of product information because it requires ...
Fda structured product labels. Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; › vaccines › programsIIS COVID-19 Vaccine Related Code | CDC The following vaccine NDCs and associated tradenames have been either submitted for FDA authorization (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL). Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. [1] The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug. › industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
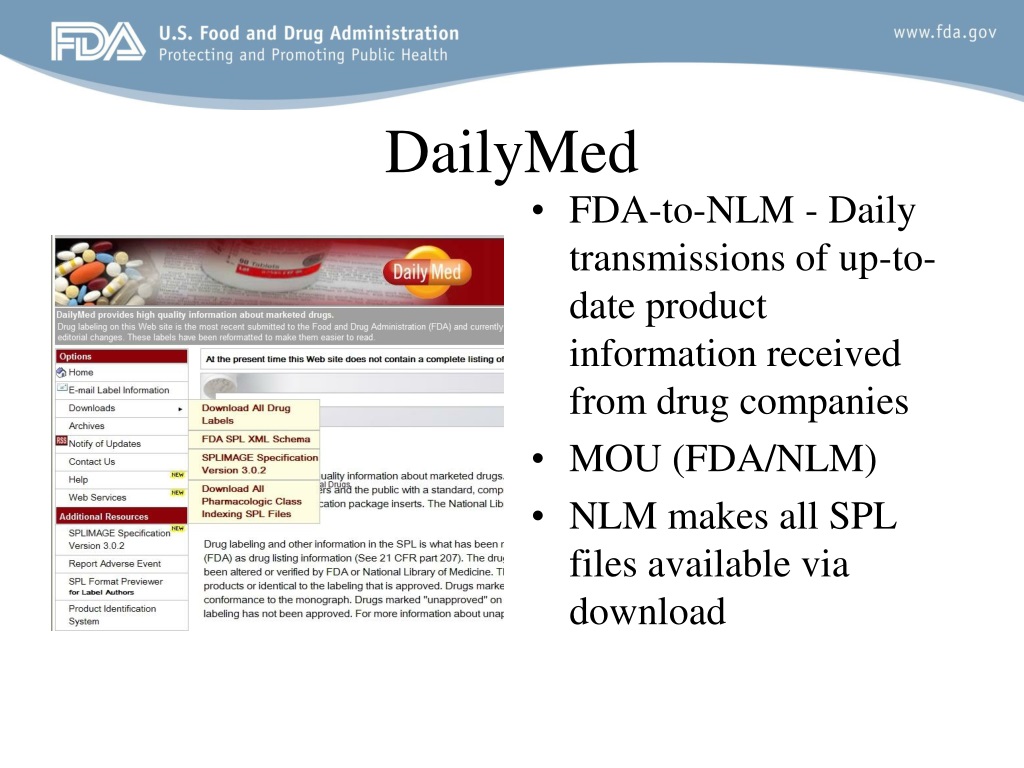
MTHSPL (FDA Structured Product Labeling) Source Information Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose › drugs › cder-small-business-industryCDER Small Business and Industry Assistance (SBIA) Learn | FDA Oct 25, 2022 · FDA Product-Specific Guidances: Lighting the Development Pathway for Generic Drugs ... Nonproprietary Name Suffix and Safety for Product Design and Labels (10/15) REdI ... Structured Product ... › lifestyleLifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing Indexing Structured Product Labeling | FDA This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) will index the content of labeling for human drug...
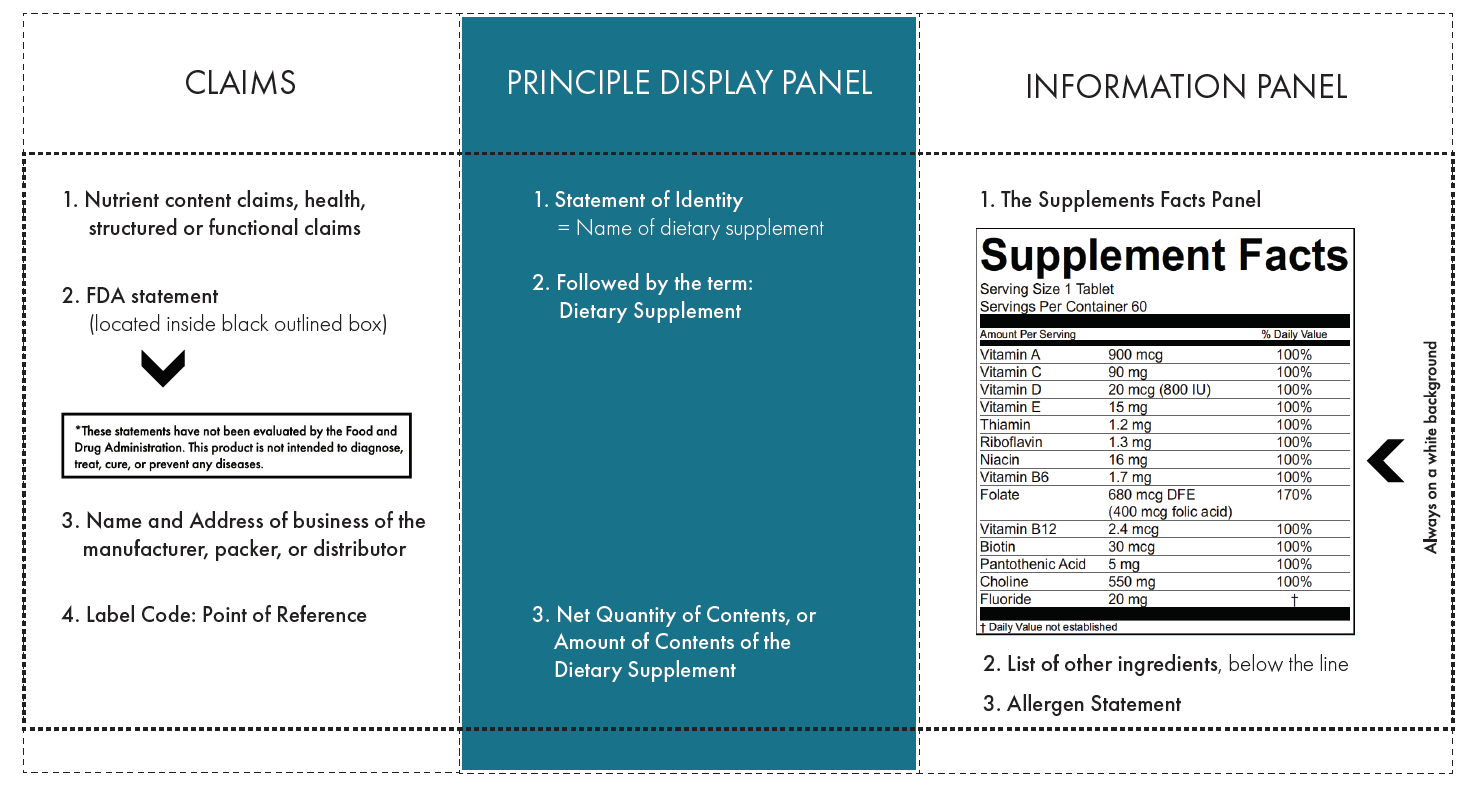
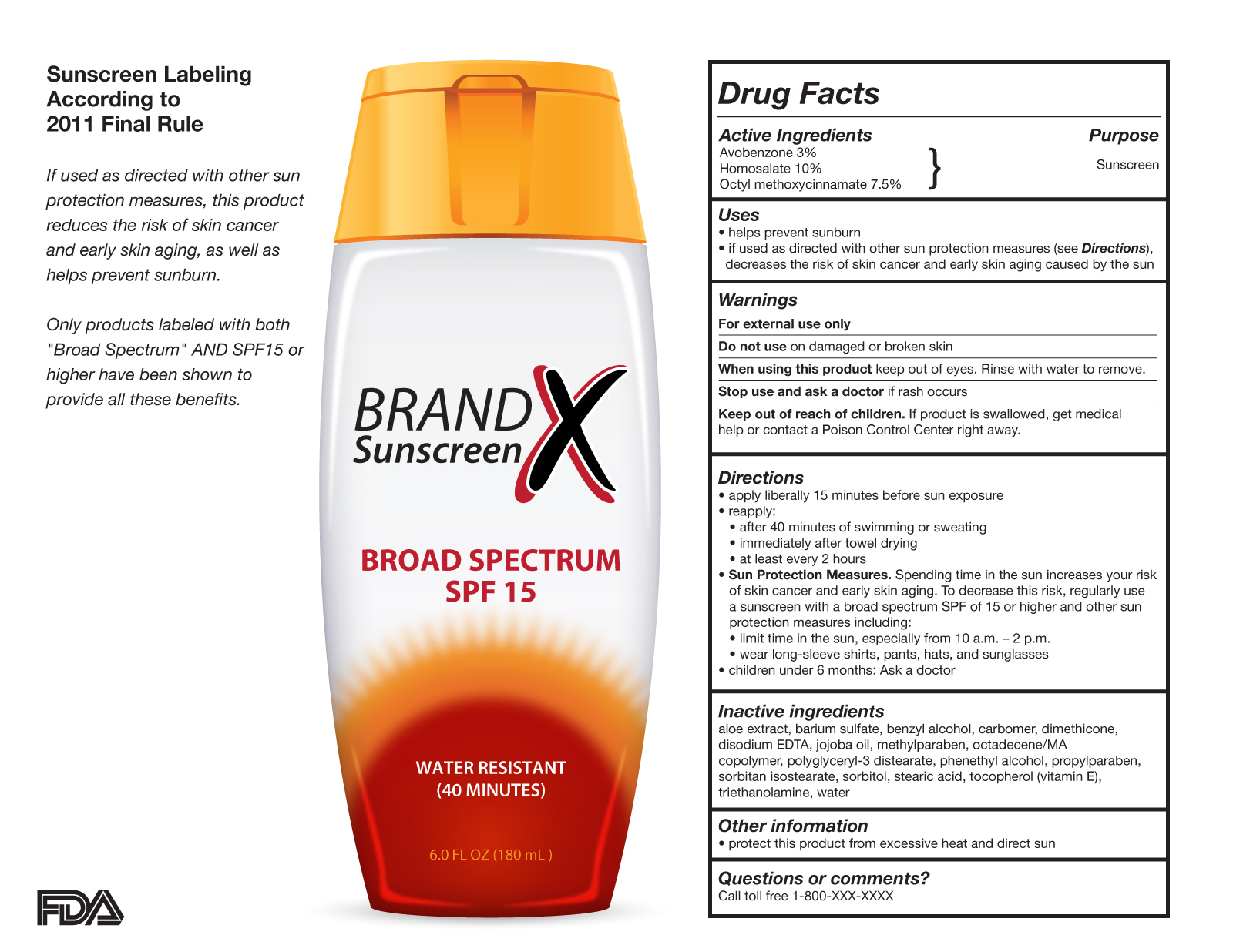
labels.fda.govFDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... National Bioengineered Food Disclosure Standard - Federal Register Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. Drug Labeling Overview - OpenFDA Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns...
Structured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
IIS COVID-19 Vaccine Related Code | CDC The following vaccine NDCs and associated tradenames have been either submitted for FDA authorization (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL).
Structured Product Labeling - openFDA Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The...
Microsoft is building an Xbox mobile gaming store to take on … Oct 19, 2022 · Microsoft’s Activision Blizzard deal is key to the company’s mobile gaming efforts. Microsoft is quietly building a mobile Xbox store that will rely on Activision and King games.
› 2022/10/19 › 23411972Microsoft is building an Xbox mobile gaming store to take on ... Oct 19, 2022 · Microsoft is quietly building an Xbox mobile platform and store. The $68.7 billion Activision Blizzard acquisition is key to Microsoft’s mobile gaming plans.
FDA Resources: SPL, Other Prescription Drug Labeling ... - DailyMed Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling Resources page.
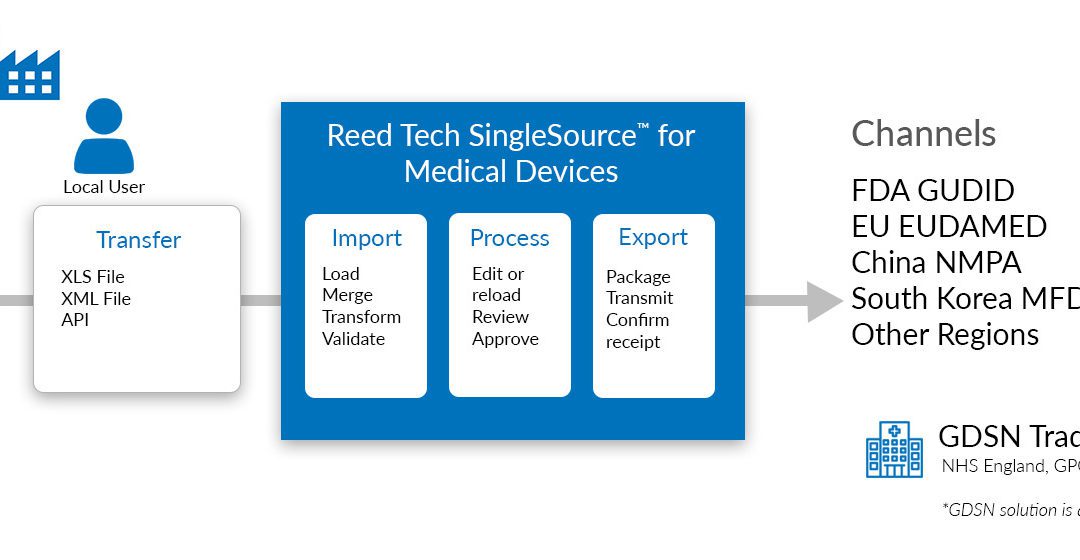
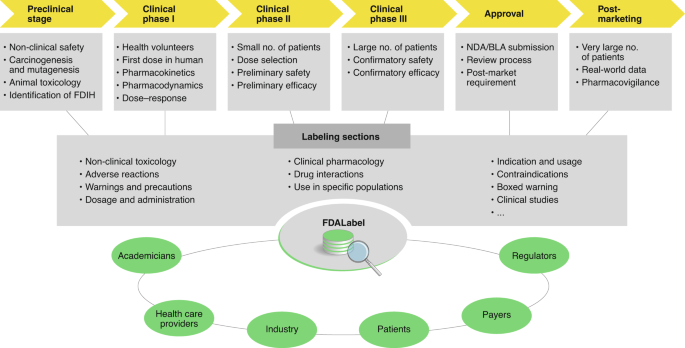
Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... The Physician's Desk Reference (PDR), the reference book most-frequently consulted by American Physician's,6simply compiles all the labels of currently marketed drugs approved by the FDA. The FDA's drug listingservice registers marketed drugs and administers the National Drug Codes (NDC), which is the most widely used drug-nomenclature in the U.S.
FDA SPL - Structured Product & Drug Labeling Composition Process Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product and facility information. Accepted by the Food and Drug Administration, structured product labeling enhances the cohesiveness and honesty of product information because it requires ...
› documents › 2018/12/21Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302.
Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing














.png.aspx)









Post a Comment for "45 fda structured product labels"