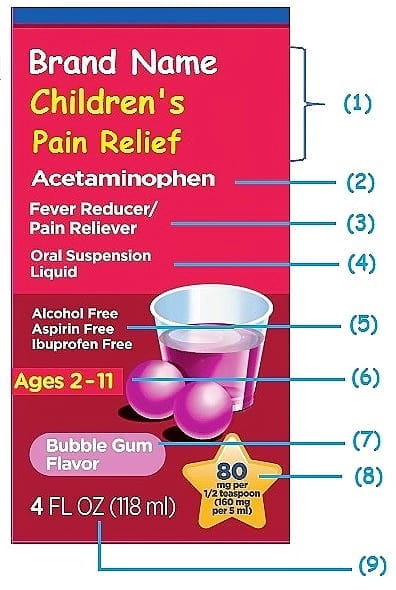
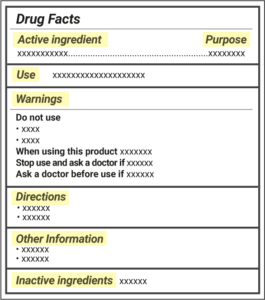
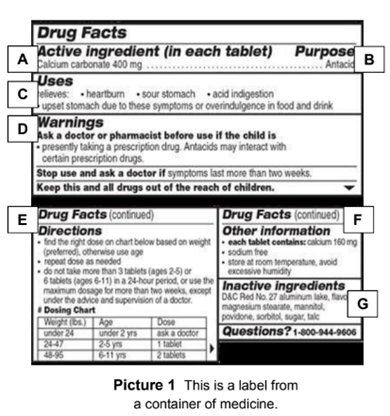
41 drug facts labels list information in the following order
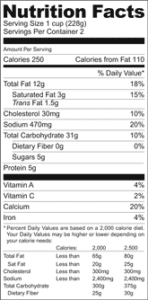
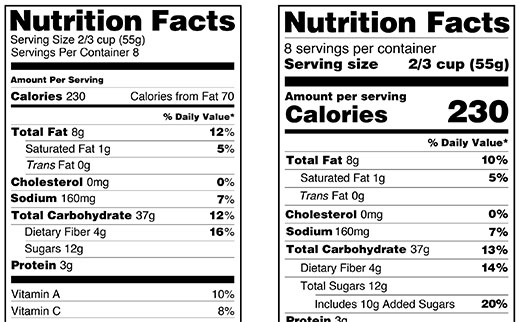
en.wikipedia.org › wiki › Nutrition_facts_labelNutrition facts label - Wikipedia The nutrition facts label is a label required on most packaged food in many countries, showing what nutrients and other ingredients are in the food. Labels are usually based on official nutritional rating systems. Most countries also release overall nutrition guides for general educational purposes. In some cases, the guides are based on different dietary targets for various nutrients than the labels on specific foods. Nutrition facts labels are one of many types of food labels required by regul Nutrition Facts table formats - Food labels - Canadian Food Inspection ... 1 serving size declaration for the food as sold, expressed in a household measure (first) and in a metric measure (second, in brackets) Complete nutrition information [absolute values ( g, mg) and where required, % Daily Value (DV)] for 1 serving of food as sold Core information is declared (Calories and 12 nutrients)
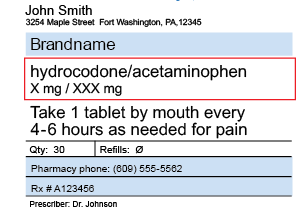
How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine.

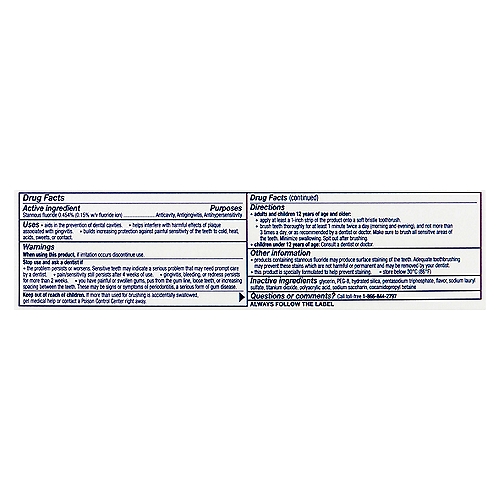
Drug facts labels list information in the following order
Medicines: packaging, labelling and patient information leaflets The regulations for labelling and patient information leaflets are set out in The Human Medicines Regulation 2012 - Part 13. Contact To discuss the content of you application, email patient ... Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons 4.5 Look-alike, Sound-alike Drug Product Names Drug labeling, Information about Drug labeling - FAQs The label must list information on contacting the manufacturer. Reports of adverse reactions to dietary supplements may be made by a healthprofessional or by consumers at 1-800-FDA-1088 or . False advertising claims may be reported to 1-877-FTC-HELP (toll free) or . User Contributions: 1 Frank
Drug facts labels list information in the following order. How important is all the information on the labels of over-the-counter ... How important is all the information on the labels of over-the-counter medicines? A: The Drug Facts label is a standardized label that appears on all over-the-counter (OTC) medicines approved by the Food and Drug Administration. It is designed to tell you the purpose of the medicine, who should take the medicine and how to take it safely. FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... How to Read Drug Labels - WebMD This part of the label will tell you the right temperature range for storing the product. It also reminds you to make sure the package's safety seal hasn't been broken before you use it, which... Learn How the Nutrition Facts Label Can Help You Improve Your Health The Nutrition Facts label on packaged foods is based on updated science and dietary recommendations for Americans. Using the label can help you choose foods for a healthy diet. The label is required on all packaged foods made in the United States and imported from other countries. The US Food and Drug Administration (FDA) issued regulations in ...
How to Read Over-the-Counter and Prescription Drug Labels - Drugwatch.com The drug label printed on prescription drug packaging doesn't tell you all the safety information for the drug, so pharmacies will give you a pharmacy information sheet. On this sheet, look for warnings or cautions, drug uses, how to use the drug, side effects, precautions, drug interactions, overdose information and how to store the drug. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 201.50 Statement of identity. (a) The label of prescription and insulin-containing drugs in package form shall bear as one of its principal features a statement of the identity of the drug. (b) Such statement of identity shall be in terms of the established name of the drug. In the case of a prescription drug that is a mixture and that has ... The Poisons Standard (the SUSMP) - Therapeutic Goods Administration (TGA) The Poisons Standard is a record of decisions on the classification of medicines and chemicals into Schedules. It also includes model provisions for containers and labels, and recommendations about other controls on medicines and chemicals. The Poisons Standard is a Legislative Instrument for the purposes of the Legislation Act 2003. How To Read Food and Beverage Labels - National Institute on Aging The ingredients in packaged food and beverage items are listed separately from (and often below) the Nutrition Facts label. This information lists each ingredient in the product by its common or usual name, and in descending order by weight.
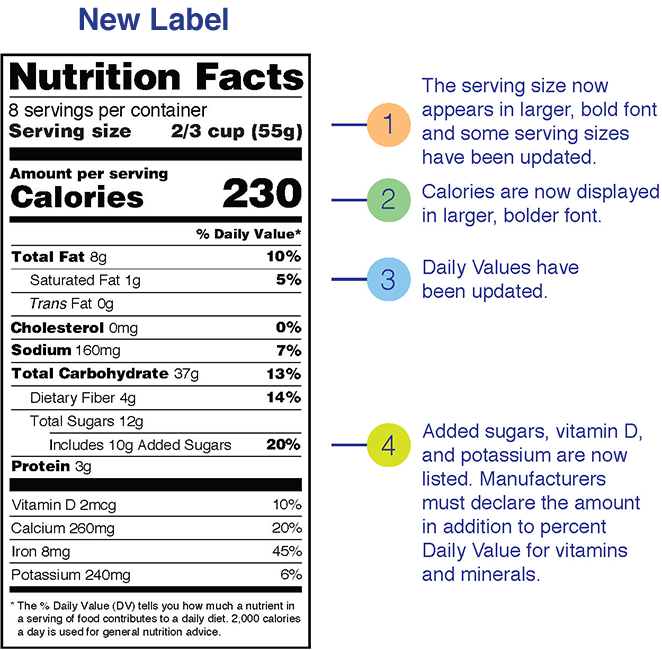
coursehelponline.comCourse Help Online - Have your academic paper written by a ... We give anonymity and confidentiality a first priority when it comes to dealing with client’s personal information. The following are some of the ways we employ to ensure customer confidentiality. We have encrypted all our databases; All our client’s personal information is stored safely; We have servers that operate 99.9% of the time Over the Counter (OTC) Drug Labels - Poison All OTC drug labels include "Drug Facts", the who, what, how, when, and why of that medicine. The Drug Facts tell you what you need to know to give the right drug, in the rightdose, to the right person, at the right time, in the right way, and for the right purpose. Food Labels | CDC - Centers for Disease Control and Prevention All the numbers on this label are for a 2/3-cup serving. This package has 8 servings. If you eat the whole thing, you are eating 8 times the amount of calories, carbs, fat, etc., shown on the label. Total Carbohydrate shows you types of carbs in the food, including sugar and fiber. Choose foods with more fiber, vitamins, and minerals. FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
National Drug Codes Explained: What You Need to Know - Drugs.com The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1, meaning that there are 4 or 5 digits for the labeler code, 4 or 3 digits for the product code and 2 or 1 digit (s) for the package code. Example NDC For example, the NDC for a 100-count bottle of Prozac 20 mg is 0777-3105-02.
Information within the Nutrition Facts table - Food labels - Canadian ... Column 1 sets out the information in the same order as in the table following B.01.401 of the FDR and also prescribes, under the heading "Nomenclature", the terms that must be used for describing this information. It is a combination of columns 1, 2 and 3 from the tables set out in the Food and Drug Regulations.
Food Label Reading - What You Need to Know Following are the recommended daily amount of some vitamins and minerals. Vitamin A % - 5000 units per day. Vitamin C % - 60mg per day. Calcium % - 1g or 1000mg per day. Iron % - 18mg per day. Riboflavin % - 1.7mg per day. Niacin % - 20mg per day. Folic Acid % - 0.4mg per day. Phosphorus % - 1.0g per day. Magnesium % - 400mg per day.
abcnews.go.com › healthHealth News | Latest Medical, Nutrition, Fitness News - ABC ... Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 201.62 Declaration of net quantity of contents. (a) The label of an over-the-counter drug in package form shall bear a declaration of the net quantity of contents. This shall be expressed in the terms of weight, measure, numerical count, or a combination or numerical count and weight, measure, or size. The statement of quantity of drugs in ...
Prescriptions- Label Flashcards | Quizlet Here is a list of all the things that need to go on a prescription label for a non-control: 1. name of pharmacy 2. address of pharmacy 3. telephone number of pharmacy 4. number of the prescription 5. date the prescription is filled 6. name of patient**** 7. name of prescriber 8. initials of the pharmacist dispensing the Rx
› it-cosmetic-drug-or-both-or-it-soapIs It a Cosmetic, a Drug, or Both? (Or Is It Soap?) | FDA For example, the drug ingredients must be listed alphabetically as "Active Ingredients," followed by cosmetic ingredients, listed in descending order of predominance as "Inactive Ingredients ...
Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
mediagazer.comMediagazer 1 day ago · Mediagazer presents the day's must-read media news on a single page. The media business is in tumult: from the production side to the distribution side, new technologies are upending the industry.
› drugs › information-consumers-andOTC Drug Facts Label | FDA Patterned after the Nutrition Facts food label, the Drug Facts label uses simple language and an easy-to-read format to help people compare and select OTC medicines and follow dosage instructions.
What's on a prescription label? - Knowledge is the best medicine Rollover A-K below to see the various part of a prescription label. * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter ...
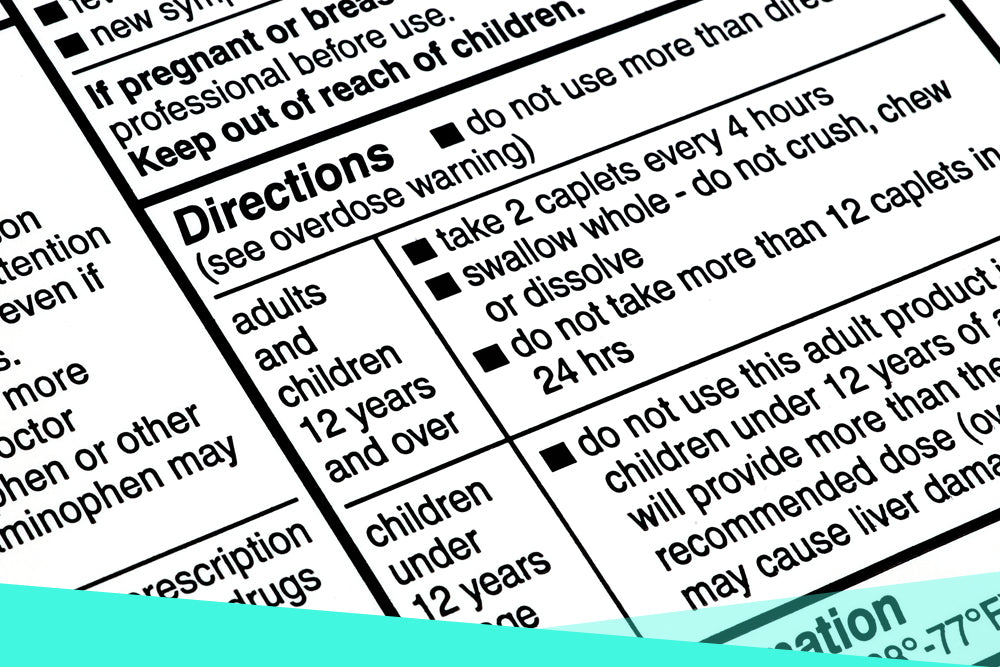
Lecture 3: Reading Medication Labels and Basic Dosage ... - cwladis Sample Medication Labels. To better understand the different pieces of information on a drug label, let's look at a few examples: Example: Trade name: Verelan Generic name: verapamil HCl. Dosage units: mg . Administration units: cap . Concentration: Each capsule is 120 mg.
› tobacco › basic_informationOutbreak of Lung Injury Associated with the Use of E ... Aug 03, 2021 · Emergency department (ED) visits related to e-cigarette, or vaping, products continue to decline, after sharply increasing in August 2019 and peaking in September.; National and state data from patient reports and product sample testing show tetrahydrocannabinol (THC)-containing e-cigarette, or vaping, products, particularly from informal sources like friends, family, or in-person or online ...
Prescription Labels and Drug Safety - Consumer Reports Prescription bottle labels. There are about 1.5 million preventable medication errors each year, according to a 2006 report from the Institute of Medicine. Roughly one-third of those mistakes ...
How to Read Over the Counter Drug Labels - Verywell Health The U.S. Food and Drug Administration (FDA) requires all over-the-counter (OTC) medications to have a Drug Facts label. This label provides basic information about a drug's ingredients, instructions for use, and important safety cautions and interactions. This information will help you to select the correct medication and to use it properly. 1
Labelling requirements for non-prescription drugs guidance document Pursuant to the Regulations, the principal display panel of an inner and outer label must normally show the following information: 1.3.1.1 The brand name of the drug product or if no brand name exists the proper or common name of the drug product, per C.01.004 (1) (a) (i);
Drug labeling, Information about Drug labeling - FAQs The label must list information on contacting the manufacturer. Reports of adverse reactions to dietary supplements may be made by a healthprofessional or by consumers at 1-800-FDA-1088 or . False advertising claims may be reported to 1-877-FTC-HELP (toll free) or . User Contributions: 1 Frank
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons 4.5 Look-alike, Sound-alike Drug Product Names
Medicines: packaging, labelling and patient information leaflets The regulations for labelling and patient information leaflets are set out in The Human Medicines Regulation 2012 - Part 13. Contact To discuss the content of you application, email patient ...















/mucinex-guaifenesin-oral-5217283-no-logo-6b5c028132dd40d2a3c512c711f0abe6.jpg)











Post a Comment for "41 drug facts labels list information in the following order"